Exclusivity of splicing factor (SF) mutations is a near-dogma in myeloid neoplasia (MN). Such exclusivity is often reflected in morphopathologic features as in the case of SF3B1 mutations associated with ringed sideroblasts. Consequences of SF mutations are hard to reconcile given the central role of RNA splicing in normal physiology and the multitude of alternative splicing changes occurring upon mutations. Historically, distinct gene isoforms play key roles in hematopoiesis and might be tumor associated.
We performed global transcript level expression analysis to identify SF specific differentially expressed isoforms associated with isoform disequilibrium. Such analysis prompted to the distinction between common and exclusive isoforms possibly discerning novel mechanistic insights in the pathogenesis of SF mutant MN. Of particular importance is the discovery of novel isoforms not expressed in normal condition.
Total RNA isolated from 100 whole bone marrows of MN patients with six SF mutations ( DDX41, PRPF8, SF3B1, SRSF2, U2AF1, ZRSR2) and healthy subjects was subjected to RNASeq library prep (Kapa RNAHyperPrep Kit with rRNA depletion). Transcript abundance was generated using spliced alignment to the genome by STAR followed by Salmon-basedquantification. We then applied bootstrapping to capture variation in abundance estimates since transcript-level quantification is amenable to variation, in contrast with gene level, due to the difficulty of unique mapping of short read sequences. Differential isoform testing was done using edgeR accounting for overdispersion due to isoform uncertainty. Mutant samples were selected with VAF >15% for better estimation of isoforms vs clonality.
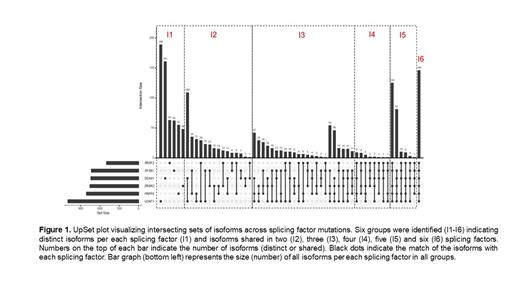
Differential SF isoform usage was defined in comparison to healthy controls. In total, 1681 unique transcripts in 1418 unique genes (positively/negatively dysregulated) at a threshold of .05 P-value and absolute logfold change of 2.0 were selected. Among patients with SF mutations, patients carrying sole U2AF1 had the highest number of distinct isoforms (n=189), which accounted for about 20% of isoforms in the entire cohort of U2AF1 mutant patients. DDX41 mutations had the second highest (n=161), while ZRSR2 had the lowest number of distinct isoforms(n=48). We categorized the features in groups I1-I6 according to the number of SF sharing isoforms. I1 indicates the group of patients carrying isoforms which where distinct per each SF ( Fig1). U2AF1I1 included genes previously reported to be associated with U2AF1 ( e.g., GNAS) and genes restricted to neutrophil degranulation. DDX41I1 was enriched in innate immune response gene isoforms including HLA-DQA1 and SAMHD1 and known genes predisposing to myeloid and blood disorders ( e.g. CSF1R, RPL15). SRSF2 isoform signature was mainly characterized by upregulation of INF-gamma and beta, IFN-induced OAS1 and TLR response pathways ( e.g., IRAK2). Specific isoforms were shared between SFs. SF3B1 shared 35 isoforms with PRPF8 and 8 with SRSF2, including Stathmin1, a gene expressed in high proliferative MN phenotypes. Different isoforms in the same gene were also associated with different groups. For instance, DDX5 isoform-219 producing a 65-residues transcript was shared between PRPF8 and SF3B1 (I2) while isoform-234 producing 614-residues transcript was shared between PRPF8, DDX41, and ZRSR2 mutants (I3). Beside the unique isoform signature per each SF, we imputed any differential isoform changes in myeloid genes often associated with SF mutations. Isoforms for the TET triad were analyzed and resulted in a 110 residues transcript (TET2-208) significantly downregulated (FDR < .05) in all SF, except SRSF2.
We then focused on the discovery of exclusive isoforms. In total, 115 unique transcripts (lncRNAs, 20; protein coding, 95) were expressed in all SF mutant patients but not expressed in healthy controls. GDF15 isoform-201 encoding a 308-residues transcript was detected in SF3B1, DDX41, and ZRSR2 mutants. LncRNAs have gained attention in regulating gene expression in MN. We found 9 novel lncRNA transcripts in DDX41, SF3B1, PRPF8, U2AF1 and ZRSR2 mutants, with one being common in all of them (ENSG00000273445 chromosome 2p11.2).
In sum, our study focusing solely on transcript level analysis describes the first isoform landscape of SF mutant MN and launches the idea that isoform analysis might shed light on the diversity and uniqueness of SF mutations.
Disclosures
Maciejewski:Regeneron: Consultancy, Honoraria; Alexion: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Omeros: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal